Myelofibrosis (MF) is the deadliest subtype of myeloproliferative neoplasm (MPN) with a median survival of approximately 5 years. Ruxolitinib, a front line therapy for JAK2V617F mutant MPN, can alleviate symptoms of the disease, but does not eliminate the malignant clone and has minimal impact on BM fibrosis and overall survival. Current mouse models do not recapitulate the clinical heterogeneity, clonal genetic composition, or morphological features of MF. Most notably, these models do not generate robust reticulin fibrosis in the bone marrow, the most significant MF pathology. This lack of clinically relevant MF models presents a major barrier to deciphering the complex genetic drivers of the disease and developing effective therapies against it.
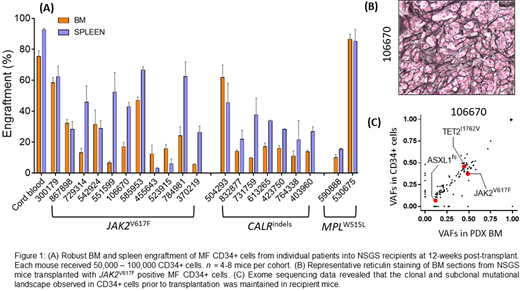
We evaluated the ability of CD34+ hematopoietic stem and progenitor cells (HSPCs) from MF patients (that contain the MPN-disease initiating population) to give rise to MF in xenotransplanted NSGS mice. >5x104 FACS-sorted CD34+ HSPCs from the peripheral blood of MF patients with JAK2V617F (n=12), CALRindels (n=7) and MPLW515L (n=2) were transplanted into sublethally irradiated (200rads) NSGS mice via X-ray guided intra-tibial injection. We observed robust engraftment of patient-derived cells at 12 weeks post-transplant regardless of their genetic background or donor patient disease severity (Fig 1A). Post-transplant, BM analysis revealed robust expansion of phenotypically defined MF HSCs relative to cord blood CD34+ control recipients, suggesting a permissive niche for MF HSCs to undergo self-renewal. Remarkably, transplantation of CD34+ cells produced other hallmarks of MF in recipient animals such as splenomegaly, thrombocytosis and most importantly BM reticulin fibrosis in all recipients (Fig 1B). We assessed the clonal architecture of engrafted human cells compared to the primary disease in the donor patients through exome sequencing of CD34+ cells prior to transplantation and hCD45+ cells from MF xenografts. We found that the clonal and subclonal mutational landscape observed in CD34+ cells prior to transplantation was maintained in recipient mice (Fig 1C), suggesting that the PDX model accurately reflects the cellular composition of the primary disease.
Intriguingly, in two of the xenografted patient samples, we identified additional mutations that were not detected in the primary patient samples using standard sequencing - namely TP53R248Q and EZH2Y663H respectively. Two years after we detected these mutations in PDXs, these MF patients transformed into sAML with acquisition of TP53R248Q and EZH2Y663H mutations. We performed droplet digital PCR and demonstrated that indeed rare pre-leukemic subclones containing these mutations were present at low levels (< 0.01% VAF) in chronic stage MF patients at least two years prior to sAML progression. These data also suggested that these rare subclones responsible for leukemic transformation expand significantly (>300 fold) under the selective pressure of transplantation in NSGS mice. Additional validation of these findings in a further six pre-sAML MF patient samples is currently ongoing. If successful, this model could be used to prospectively identify rising leukemic clones in chronic stage MF patients, which are below the level of detect of standard sequencing as a mechanism to stratify such patients for more aggressive treatments. While sequencing can identify ultra-rare variants, it cannot discern their functional potential for sAML transformation, which is the advantage of this approach.
Finally, we harnessed this system for pre-clinical studies, initially focusing on inhibiting the JAK/STAT signaling pathway. Ruxolitinib treatment in MF PDXs produced remarkably similar phenotypes as observed in patients. We observed a small, but significant reduction in engraftment of MF cells in the BM and a sharp reduction in spleen size in Ruxolitinib-treated group compared to vehicle control. Ruxoltinib treatment however did not reduce the frequency of MF HSCs, the disease initiating population or lessen the degree of reticulin fibrosis. These data suggest that this system can be used as a reliable, clinically-relevant drug screening platform. Taken together, we offer the field a critical, previously missing biologically relevant screening system for validation of MPN drug targets identified in cell lines or genetic mouse models prior to moving forward into clinical trials.
Oh:Blueprint Medicines: Consultancy; Celgene/BMS: Consultancy; Constellation: Consultancy; CTI Biopharma: Consultancy; Disc Medicine: Consultancy; Gilead Sciences: Consultancy; Incyte Corporation: Consultancy; Kartos Therapeutics: Consultancy; Novartis: Consultancy; PharmaEssentia: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.